
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
The Expert Market Research report, titled “Tiotropium (Spiriva) Manufacturing Plant Project Report 2026 Edition: Industry Trends, Capital Investment, Price Trends, Manufacturing Process, Raw Materials Requirement, Plant Setup, Operating Cost, and Revenue Statistics” includes various aspects that are critical for establishing a tiotropium (Spiriva) plant. These include infrastructure requirements, transportation requirements, utility specifications, and financial and economic analysis, among others.
The demand for tiotropium (Spiriva) is increasing due to increasing prevalence of chronic obstructive pulmonary disease (COPD). As per industry reports, the number of COPD cases will reach approximately 600 million by 2050, reflecting a 23% increase from 2020 levels. In 2024, the estimated prevalence of COPD among individuals aged 40 years and older is around 12.64%, highlighting a significant public health concern. This increase is notably pronounced among women, with projections suggesting a 47.1% rise in female COPD cases by 2050. Also, in low- and middle-income countries (LMICs), the burden of COPD is expected to grow at a much faster rate than in high-income countries (HICs), which is further driving demand for effective treatments like tiotropium.
Other elements to consider while establishing a tiotropium (Spiriva) plant include raw material sourcing, workforce planning, and packaging. The production of tiotropium (Spiriva) relies on several key raw materials, such as tiotropium bromide monohydrate, lactose monohydrate, magnesium stearate, and silica gel. Tiotropium bromide monohydrate is the primary active pharmaceutical ingredient (API) and is synthesised as a quaternary ammonium salt and exists as a white to yellowish-white crystalline powder. Its unique chemical structure allows it to function effectively as a long-acting anticholinergic agent, specifically targeting muscarinic receptors in the airways to induce bronchodilation.
Another essential raw material is lactose monohydrate, which acts as a carrier for the tiotropium powder, aiding in proper delivery during inhalation and improving aerosolisation properties. Each Spiriva capsule contains approximately 5.5 mg of lactose monohydrate. Other excipients such as magnesium stearate and silica gel play crucial roles in the manufacturing process; magnesium stearate acts as a lubricant to prevent clumping of powders, while silica gel serves as an anti-caking agent to maintain powder flowability and prevent moisture absorption. These raw materials are vital for producing Spiriva in accordance with pharmaceutical standards.
Moreover, to help stakeholders determine the economics of a tiotropium (Spiriva) plant, project funding, capital investments, and operating expenses are analyzed. Projections for income and expenditure, along with a detailed breakdown of fixed and variable costs, direct and indirect expenses, and profit and loss analysis, enable stakeholders to comprehend the financial health and sustainability of a business. These projections serve as a strategic tool for evaluating future profitability, assessing cash flow needs, and identifying potential financial risks.
Tiotropium bromide is a long-acting bronchodilator primarily used for the management of chronic obstructive pulmonary disease (COPD) and asthma. Marketed under the trade name Spiriva, it functions as a muscarinic antagonist, providing sustained bronchodilation through once-daily inhalation. With a bioavailability of 19.5% and a half-life of 5–6 days, tiotropium is metabolised in the liver and is predominantly excreted unchanged.
It is classified as a prescription-only medication in several regions, including Australia, the UK, and the US, and has a favourable safety profile, with dry mouth being the most common side effect. Tiotropium was developed in the late 1990s and received FDA approval in 2004. It was the first long-acting muscarinic antagonist (LAMA) available for COPD treatment. Its introduction marked a significant advancement in bronchodilator therapy, offering improved lung function and reduced exacerbations compared to previous treatments.
Tiotropium bromide, the active ingredient in Spiriva, is a non-chiral quaternary ammonium compound with a molecular formula of C19H22BrN3O4S2·H2O and a molecular mass of 490.4 g/mol. It appears as a white or yellowish-white powder that is sparingly soluble in water but soluble in methanol. The drug exhibits a long elimination half-life of 5–6 days and is primarily metabolised in the liver via CYP2D6 and CYP3A4 pathways. Tiotropium is delivered through inhalation, ensuring targeted action in the lungs for effective bronchodilation.
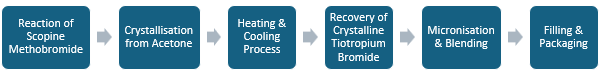
The manufacturing process of tiotropium bromide (Spiriva) begins with the reaction of scopine methobromide with an anhydride compound in acetonitrile, which is heated under reflux for approximately four hours. Following this reaction, the mixture undergoes crystallisation from a methanol/acetone mixture, where it is heated to 50-60°C and then cooled to -6 to -14°C to recover crystalline tiotropium bromide. The crystalline product is then micronised to achieve the desired particle size distribution. Next, the micronised tiotropium bromide is blended with lactose monohydrate, and the resulting powder blend is filled into capsules. Finally, these capsules are packaged with the HandiHaler inhalation device, resulting in the final product, Spiriva HandiHaler, which is used for the delivery of tiotropium bromide as an inhalation powder.
Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
The synthesis of tiotropium involves several chemical reactions, such as:
1. Synthesis of Tropenol
The synthesis of tiotropium begins with the formation of the tropenol intermediate. Tropenol can be made through a multi-step process starting from tropinone.
Chemical Reaction - Tropenol
C8H13NO (Tropinone) + H2 → C8H15NO (Tropanol)
Tropinone is reduced using a suitable reducing agent like sodium borohydride to yield tropanol.
N-Methylscopolamine is a key intermediate in the synthesis of tiotropium. It can be extracted through a series of chemical reactions involving the quaternisation of tropenol with methyl bromide.
2. Quaternisation:
C8H15NO (Tropanol) + CH3Br (Methyl Bromide) → C9H18NO (N-Methylscopolamine) + Br-
The tropenol intermediate reacts with methyl bromide, forming the quaternary ammonium compound N-Methylscopolamine.
3. Formation of Tiotropium
Tiotropium is formed by combining N-Methylscopolamine with dithienylglycolic acid, followed by bromination.
Chemical Reactions - Tiotropium
C9H18NO+ (N-Methylscopolamine) + C10H8O3 (Dithienylglycolic Acid) → Tiotropium Precursor
Tiotropium Precursor + Br2 → C19H22BrNO4 (Tiotropium Bromide)
The precursor is then brominated to yield tiotropium bromide.
4. Purification and Crystallisation
The crude tiotropium bromide is purified through crystallisation or other purification methods to remove any impurities, ensuring the final product meets pharmaceutical-grade standards.
5. Formulation into Inhaler Form
The purified tiotropium bromide is formulated into a powder for inhalation or other dosage forms. It is blended with carriers and other excipients, ensuring uniform distribution and accurate dosing in the final product.
6. Final Product
The final product is tiotropium bromide, packaged in capsules or other forms suitable for use in inhalers, such as the Spiriva HandiHaler or Respimat devices. It provides long-term bronchodilation for patients with COPD.
The tiotropium (Spiriva) market is primarily driven by its applications in managing chronic obstructive pulmonary disease (COPD) and asthma, conditions affecting millions globally. As a long-acting bronchodilator, tiotropium is favoured for its once-daily dosing, which enhances patient compliance and improves quality of life by reducing symptoms and exacerbations. The increasing prevalence of COPD, projected to rank as a leading cause of morbidity and mortality, further fuels market growth. Additionally, the drug's cost-effectiveness compared to alternatives like ipratropium and its inclusion in treatment guidelines solidify its position as a first-line therapy for respiratory conditions in the market.
A detailed overview of production cost analysis that evaluates the manufacturing process of tiotropium (Spiriva) is crucial for stakeholders considering entry into this sector. Furthermore, stakeholders can make informed decisions based on the latest economic data, technological innovations, production process, requirements of raw materials, utility and operating costs, capital investments by major players, pricing strategies, and profit margins. For instance, the introduction of PUR0200 formulation represents a notable advancement in tiotropium delivery, as it uses a pressurised metered-dose inhaler (pMDI) system. This formulation has demonstrated improved chemical stability and enhanced lung deposition compared to traditional inhalers like Spiriva HandiHaler. The incorporation of citric acid in the formulation has been shown to stabilise tiotropium bromide, reducing degradation, and ensuring consistent dosing over time. This competition can lead to increased marketing efforts and potential price adjustments from Spiriva manufacturers to maintain market share.
Below are the sections that further detail the comprehensive scope of the prefeasibility report for a tiotropium (Spiriva) production plant:
Market Dynamics and Trends: Growth factors such as increasing prevalence of respiratory diseases and the impact of generic competition significantly affect market conditions in the tiotropium (Spiriva) sector. Conditions like asthma are on the rise globally, largely due to worsening of AQI levels amid growing pollution. The World Health Organization estimates that approximately 334 million people worldwide suffer from asthma, leading to a greater need for effective treatments like Spiriva.
The impact of generic competition has also started to influence the market significantly. With the expiration of patent protections in various regions, generic versions of tiotropium are entering the market, leading to price competition, and potentially reducing sales for branded products like Spiriva. For example, Boehringer Ingelheim reported a 16% decline in Spiriva sales due to increased competition from generics in certain markets. This shift necessitates strategic adjustments by manufacturers to maintain market share and profitability. Understanding these trends helps businesses align their production plans with demands and trends in the tiotropium (Spiriva) market.
Profiling of Key Industry Players: Leading manufacturers like Boehringer Ingelheim and Lupin Limited are included in the tiotropium (Spiriva) report. Recently, in October 2023, Lupin received approval from the U.S. FDA for its generic version of tiotropium bromide inhalation powder, marking a significant milestone as it is the first generic equivalent of Spiriva HandiHaler approved in the United States. This product is manufactured at Lupin's facility in Pithampur, India. The launch is expected to provide an alternative treatment option for the millions of adults affected by COPD in the United States, further contributing to market dynamics in the respiratory sector.
Economic Analysis: Capital expenditure (CAPEX) analysis provides stakeholders the knowledge about required investments in advanced technologies, efficient machinery, and necessary infrastructure. Investing in high-capacity mixing equipment, such as a continuous mixer or high-shear mixer, can improve production efficiency by 20-30%. Investing in energy-efficient systems, such as combined heat and power (CHP) systems could reduce energy consumption by up to 30%, as these systems use waste heat from production processes to generate electricity and provide heating.
Fluctuations in the prices of tiotropium (Spiriva) are influenced by several key factors, including the costs of essential raw materials, market competition, insurance coverage, and manufacturer pricing strategies. Currently, the price for Spiriva HandiHaler (18 mcg inhalation capsule) can range from approximately USD 116.28 for a 5-capsule supply to USD 1,627.00 for a 90-capsule supply. Generic versions of tiotropium are also available, with prices starting around USD 479.54 for a 30-capsule supply. Without insurance, the average cost can be about USD 791.11, although discount programs may reduce this to approximately USD 191.46.
Key factors influencing these price fluctuations include the cost of raw materials used in production, which can directly impact manufacturing expenses. Additionally, the entry of generic medications into the market often drives down prices due to increased competition. Furthermore, Boehringer Ingelheim, the manufacturer of Spiriva, has introduced a price cap of USD 35 per month for eligible patients with private insurance starting June 1, 2024, which aims to enhance access to this essential medication and could further affect pricing dynamics in the market.
Establishing a tiotropium (Spiriva) manufacturing facility requires a comprehensive financial investment that encompasses various elements critical to the project's success. The following sections detail these components:
Projected profit margins and effective product pricing strategies improve overall profitability. Manufacturers might target a profit margin of around 20-30%, achieved through strategic pricing based on raw material costs and prevailing market demand. Effective pricing strategies should consider fluctuations in raw material prices and competitive positioning within the market.
The establishment of a tiotropium (Spiriva) manufacturing facility must comply with various regulatory frameworks that govern production standards. One of the primary frameworks is the European Directive 2001/83/EC, which regulates the marketing authorisation of medicinal products in the European Union. This directive mandates that companies submit a comprehensive dossier containing administrative, quality, pre-clinical, and clinical data to demonstrate the safety and efficacy of the drug before it can be marketed. Additionally, compliance with Good Manufacturing Practices (GMP) is critical, as outlined in Directive 2003/94/EC, which ensures that products are manufactured consistently and controlled according to quality standards across all production sites.
In the United States, the Federal Food, Drug, and Cosmetic Act (FDCA) governs the approval process for drugs like Spiriva. The U.S. Food and Drug Administration (FDA) requires a New Drug Application (NDA) that includes extensive clinical trial data supporting the drug's safety and efficacy for its intended use. Furthermore, both the FDA and Health Canada have specific guidelines for inhalation products, which include additional requirements for demonstrating that generic inhalers are interchangeable with brand-name products. Compliance with these regulations not only ensures legal operation but also enhances product safety and marketability.
This prefeasibility report aims to equip potential investors and existing manufacturers with crucial insights to make informed decisions in the tiotropium (Spiriva) industry.




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Basic Plan
USD 5,699
USD 4,844
Get Startedtax inclusive*
Raw Material and Product Specification, Raw material consumption, Process flow diagram
Machinery Cost, Working Capital
Utilities consumption, Operating cost, Overheads, Financing Charges, GSA , Packaging
Premium Plan
USD 6,799
USD 5,779
Get Startedtax inclusive*
Key Processing Information, Capital Investment Analysis, Conversion Cost Analysis
Raw material consumption and prices, Utilities consumption breakdown, By-Product Credit, Labour Charges Breakdown
Land and Site Cost, Equipment Cost, Auxiliary Equipment Cost, Contingency, Engineering and Consulting Charges
Enterprise Plan
USD 8,899
USD 7,564
Get Startedtax inclusive*
Key Processing Information, Capital Investment Analysis, Conversion Cost Analysis, Variable Cost Breakdown, Investing Cost Breakdown,
Breakdown of machinery cost by equipment, Auxiliary Equipment Cost, Piping, Electrical, Instrumentation
Cost of Construction, Plant Building, Site Development Charges
Land Cost, Development Charges
Dynamic Spreadsheet (Unlocked)
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*

Basic Plan
USD 5,699
USD 4,844
Key Processing Information
Raw Material and Product Specification, Raw Material Consumption, Process Flow Diagram
Capital Investment Analysis
Machinery Cost, Working Capital
Conversion Cost Analysis
Utilities Consumption, Operating Cost, Overheads, Financing Charges, GSA , Packaging

Premium Plan
USD 6,799
USD 5,779
All Contents of Basic Report
Key Processing Information, Capital Investment Analysis, Conversion Cost Analysis
Variable Cost Breakdown
Raw Material Consumption and Prices, Utilities Consumption, Breakdown By-Product Credit, Labour Charges Breakdown
Investing Cost Breakdown
Land and Site Cost, Equipment Cost, Auxiliary Equipment Cost, Contingency, Engineering and Consulting Charges

Enterprise Plan
USD 8,899
USD 7,564
Includes all Report Content
Key Processing Information, Capital Investment Analysis, Conversion Cost Analysis, Variable Cost Breakdown, Investing Cost Breakdown,
Equipment Cost Breakdown
Breakdown of Machinery Cost By Equipment, Auxiliary Equipment Cost, Piping, Electrical, Instrumentation
Land and Construction Cost Details
Land Cost, Development Charges, Cost of Construction, Plant Building, Site Development Charges
Dynamic Excel Cost Model
Dynamic Spreadsheet (Unlocked)
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share