
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
The allogeneic CAR T-cell market was valued at USD 1.01 Billion in 2025. It is expected to grow at a CAGR of 15.60% for the forecast period of 2026-2035 and attain a market value of USD 4.30 Billion by 2035. The market value is impacted by increased technical advancements as well as the rising incidence of cancer. The need for cutting-edge technologies owing to clearly offer broader patient access as compared to the autologous therapies due to premanufactured and off-the-shelf products is driving the rise in the number of patents in this industry.
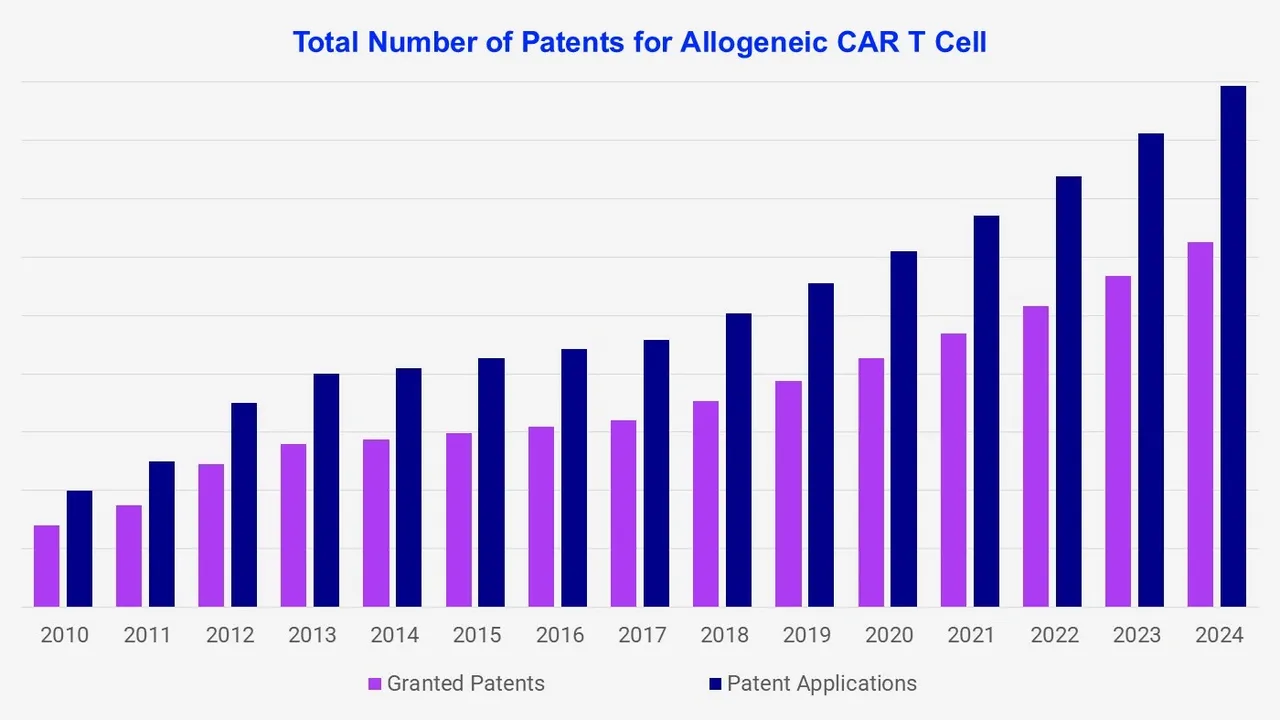
The global allogeneic CAR T-cell patent landscape report provides a comprehensive and in-depth analysis of the patents in this growing industry. The key sections captured in the report for global allogeneic CAR T-cell include patent distribution by time, geographical coverage, top IP player profiles, technological segmentation, and patent valuation.
A thorough examination of the patent portfolios of key players, covering aspects such as the number of patents, types of technologies patented, and trends over time. A detailed description of the companies granted with patents, analysis of the distribution of patents geographically, and information on key regions where patent filings are concentrated. The breakdown of patents by technical segments is provided, giving more clarity on the specific areas of innovation within allogeneic CAR T-Cell technologies.
For instance, CRISPR/Cas9 gene editing technology permits exact T cell modification for the need of inserting the CAR gene and conditionally editing other genes to increase safety, improve persistence or lessen immunological rejection.
Allogeneic CAR T-Cell (chimeric antigen receptor) therapy is a state-of-the-art cancer treatment that uses T cells from healthy donors. Thanks to genetic engineering, these T-cells express receptors that specifically target antigens in cancer cells. When compared to autologous CAR-T therapy (which makes use of the patient’s cells), this over-the-counter therapy enables quicker and more extensive treatment. Avoiding graft-versus-host disease (GvHD), which is commonly treated with gene editing, is one of the major.
Allogeneic CAR T-cell continues to evolve, driven by decreasing the cost of therapy making it more affordable and accessible. Its ability to deliver effective therapeutic results in combination with other therapies against cancer and other immune diseases, which is a driving factor for the market in forecasted period.
Technical Advancements and Product Launches are Expected to Augment the Market Growth
Universal donor CAR-T cells are engineered from healthy donors and designed to be used across multiple patients, providing an off-the-shelf solution. Allogeneic CAR T-cell treatment options are getting advanced by the research and development of market leaders to treat diseases like cancer.
For instance, in January 2023, a well-known company, Allogene Therapeutic’s ALLO-501, an Allogeneic CAR T-cell therapy targeting CD19 (a protein present on the surface of B-cell tumors) showed promising results in its phase-1 clinical trial when used for the treatment of non-Hodgkin lymphoma. The ability to develop a ready-to-use therapy lowers production time and costs significantly in comparison to patient-specific (autologous) CAR-T treatments, increasing accessibility and scalability of the treatment.
Gene Editing Technologies to Enhance the Efficacy and Safety is Expected to Propel the Allogeneic CAR-T Cell Industry Growth
Enhancing the safety and efficacy of allogeneic CAR T-cell therapies requires the application of CRISPR /Cas9 and other gene editing technologies. These technologies enable the precise modification of T-cells to target cancer and eliminate T-cell receptors (TCRs) that cause graft-versus-host disease (GvHD) more efficiently. Consequently, there is a decreased possibility of adverse reactions and safer, more efficient treatments.
The report will cover the following sections in detail:
Breakup by Type
Breakup by Target Indication
Breakup by Target Antigen
Allogeneic CAR T-Cell investigations have been conducted with the chosen patent families based on the technology to which they pertain. This patent landscape report features various technologies present in the allogeneic CAR T-Cell market including zinc finger nucleases (ZFN), next-generation sequencing (NGS), clustered regularly interspaced short palindromic repeats (CRISPR)/Cas9 and transcription activator-like effector nucleases (TALEN). Next-generation sequencing (NGS) is used in allogeneic CAR-T Cell therapy to perform comprehensive quality control and genetic screening of engineered T-cells. This ensures that the modified T-cells meet all relevant safety and efficacy standards are free of accidental alterations and have the necessary genetic modifications before being infused into patients.
The detailed technological data will be provided for all specified segments classified in this report.
Over half of the allogeneic CAR T-Cell patent filings are in the United States, underscoring its role as the global hub for Allogeneic CAR T-cell development. The region has a strong biotech industry, funding ecosystem, and presence of leading CAR-T companies (e.g., Allogene Therapeutics, Precision BioSciences). The FDA’s evolving regulatory framework for cell therapies may also encourage filings. The EU remains a critical secondary market with steady filings, probably targeting Germany, the UK, and France during national phase entry. A significant portion of filings suggest active European interest. Regulatory harmonization through the EPO and access to multiple EU markets make this a valuable region.
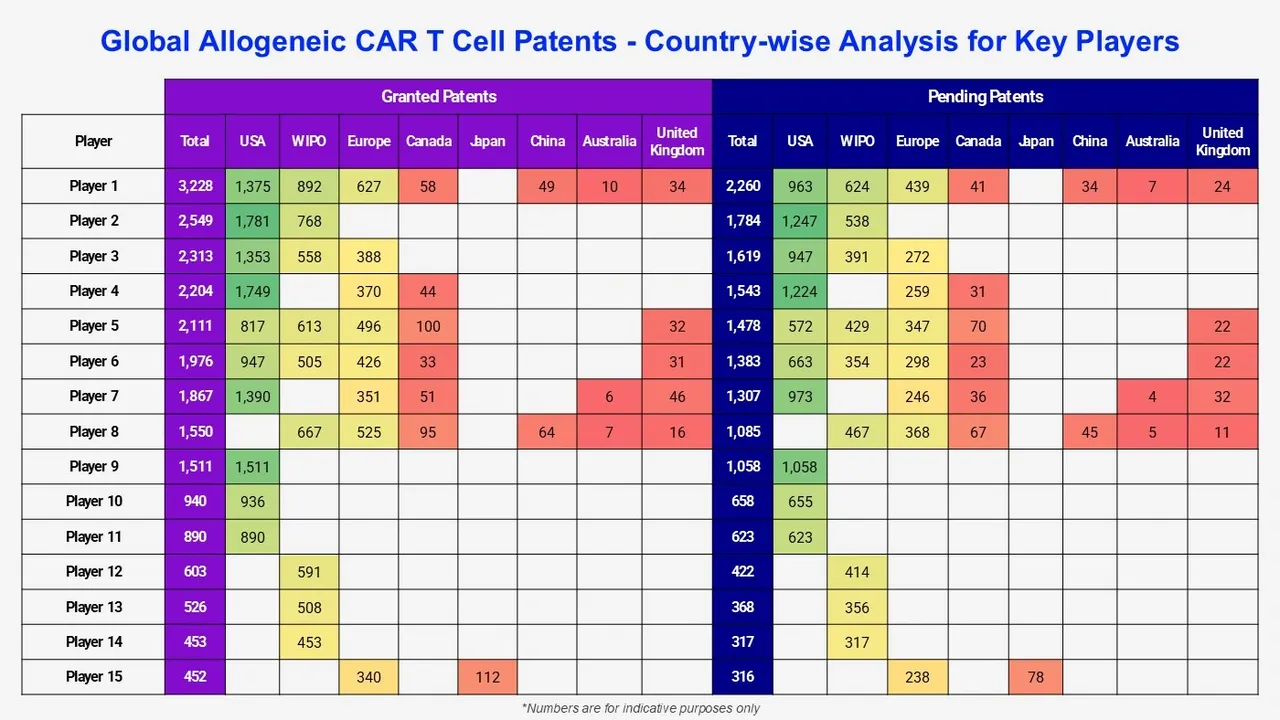
This study includes an analysis of key players in the global allogeneic CAR T-Cell patent landscape, detailing their patent portfolios, patent issuance trends over time, geographic coverage, active patents in various technical segments, and financial analysis. After this patent profile overview, we look at the technological content found in their main patents.
Among the players with allogeneic CAR T-cell patent families, pre-existing new entrants have been identified. These companies can be either established companies or startups developing their first technology in the field. These innovative companies can become a future key player in healthcare, attracting investments from major corporations.
Some of the major companies mentioned in this report (a non-exhaustive list) are as follows:
A biopharmaceutical company renowned for developing innovative cancer, cardiovascular, and immunology treatments. BMS is working hard to improve its Allogeneic CAR-T Cell (chimeric antigen receptor) therapy pipeline. The company wants to create off-the-shelf CAR-T cell treatments that will improve accessibility and efficacy for cancer patients by employing cutting-edge gene editing technologies.
Compugen LTD
Leading therapeutic development company focused on mmune-oncology discovery at the clinical stage. This company uses its computational discovery platforms and in-depth understanding of the tumour microenvironment to collaborate with other biopharmaceutical companies on cutting-edge therapies, such as Allogeneic CAR-T cell therapies, to improve cancer treatment. Development of biological drugs and the identification of new drug targets are its two main areas of expertise.
Other players in the market include Artiva Biotherapeutics Inc., Poseida Therapeutics, Inc., Nanjing Beiheng Biological Technology Co., Ltd., and NantCell, Inc., among others.
Studying the intellectual property (IP) position and strategy of players in the global allogeneic CAR T- cell patent landscape report is crucial for several reasons:
Global CAR-T Cell Therapy Market
Global Cell and Gene Therapy Market




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
| Scope of the Report | Details |
| Analysis by Type |
|
| Target Indication |
|
| Target Antigen |
|
| Key Players Mentioned |
|
| Geographies Covered |
|
Mini Report
One User
USD 2,699
USD 2,429
tax inclusive*
Single User License
One User
USD 4,299
USD 3,869
tax inclusive*
Five User License
Five User
USD 5,799
USD 4,949
tax inclusive*
Corporate License
Unlimited Users
USD 6,999
USD 5,949
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share