
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
According to the National Organisation for Rare Disorders, warm autoimmune hemolytic anemia (wAIHA) is the most common subtype of autoimmune hemolytic anemia, affecting approximately 1 to 3 per 100,000 people every year. The disease can occur at any age, therefore, there is a significant focus on designing effective drugs that help in successfully managing and treating the disease.
Major companies involved in the warm autoimmune hemolytic anemia pipeline drugs market include Janssen Research & Development, LLC, Incyte Corporation, Rigel Pharmaceuticals and Sanofi.
Leading drugs currently under pipeline include Obexelimab, ALXN1830, M281 and Rilzabrutinib, among others.
Regulatory authorities such as the United States FDA and EMA play an essential role in the warm autoimmune hemolytic anemia emerging drugs pipeline as they are offering breakthrough designations to manage the condition.
The Warm Autoimmune Hemolytic Anemia Drug Pipeline Report by Expert Market Research gives comprehensive insights into ongoing warm autoimmune hemolytic anemia clinical trials. It covers various aspects related to the details of warm autoimmune hemolytic anemia drugs under development. The report includes the analysis of over 100 pipeline drugs and 50+ companies. The warm autoimmune hemolytic anemia pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials including their adverse effects on patients suffering from warm autoimmune hemolytic anemia.
The detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration and ongoing warm autoimmune hemolytic anemia pipeline development activities are covered. Moreover, warm autoimmune hemolytic anemia collaborations and commercial assessments are offered, helping stakeholders to make informed decisions.
Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
Warm autoimmune hemolytic anemia is an autoimmune condition that leads to premature death of red blood cells in the body. It is the most common type of autoimmune hemolytic anemia. Normally, red blood cells have a lifespan of 120 days before they are ultimately destroyed by the spleen. However, individuals affected by this condition witness sudden death of RBCs at a rapid pace and to an extent wherein the body cannot compensate for the drastic loss. Symptoms may include tiredness, dizziness, jaundice or heart palpitations.
The first-line warm autoimmune hemolytic anemia treatment involves corticosteroids. These drugs are successful in about 65-70% of the patients. In relapsed cases, splenectomy, rituximab or other immunosuppressive drugs are advised. In December 2023, researchers at the American Journal of Hematology revealed that Fostamatinib, a spleen tyrosine kinase inhibitor, previously used for the treatment of patients with chronic immune thrombocytopenia in the United States can be found successful in managing warm antibody autoimmune hemolytic anemia (wAIHA) effectively. Such initiatives aimed at developing high-efficacy drugs are amongst major trends, impacting the drug pipeline outlook positively.
This section of the report covers the analysis of warm autoimmune hemolytic anemia drug candidates based on several segmentations including:
By Phase
EMR’s pipeline assessment report covers 50+ drug analyses based on phase.
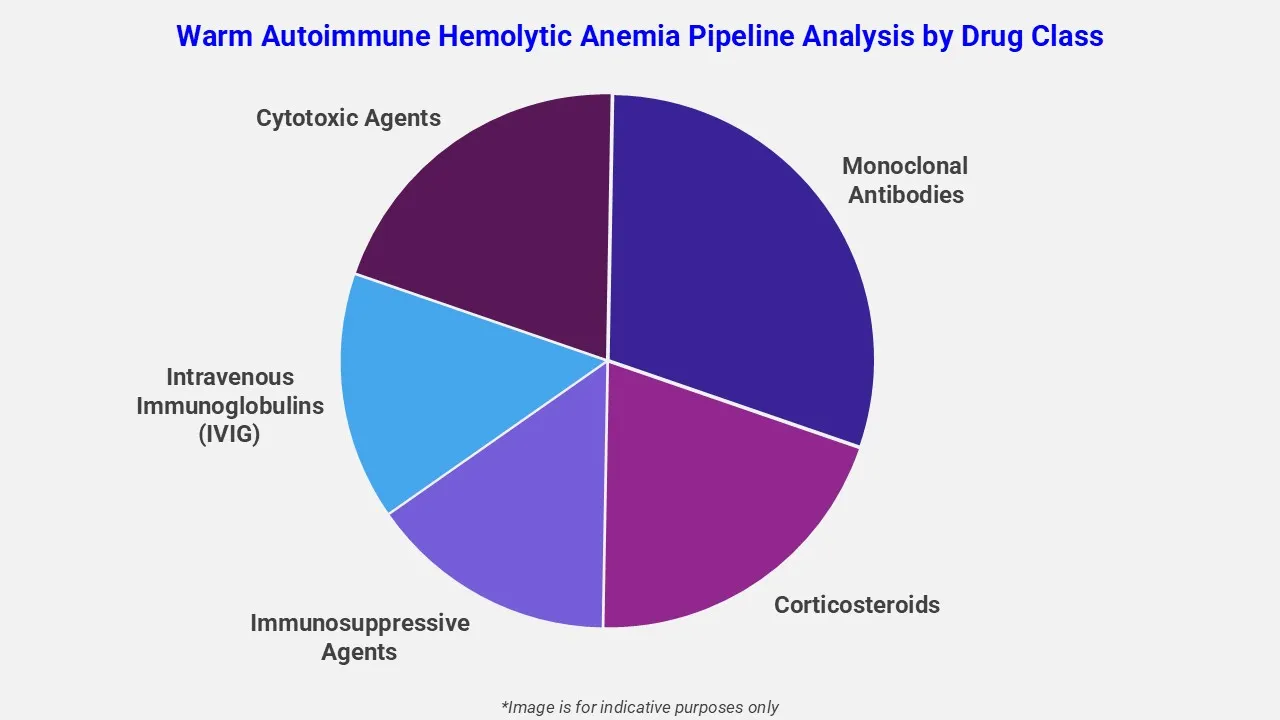
By Drug Class
EMR’s warm autoimmune hemolytic anemia therapeutic assessment report covers 50+ drug analyses based on drug classes:
By Route of Administration
EMR’s pipeline assessment report covers 50+ drug analyses based on the route of administration.
The report covers phase I, phase II, phase III, phase IV, and early phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, phase II covers a major share of the total clinical trials for warm autoimmune hemolytic anemia. There are around 20 drugs in phase II of warm autoimmune hemolytic anemia drugs.
The drug molecules categories covered under warm autoimmune hemolytic anemia pipeline analysis include corticosteroids, immunosuppressive agents, intravenous immunoglobulins (IVIG), cytotoxic agents and monoclonal antibodies. These drugs can be used in combination, based on the patient’s response to treatment, symptoms and severity of the condition.
The EMR warm autoimmune hemolytic anemia drug pipeline report covers the profile of key companies involved in clinical trials and their drugs under development. Below is the list of a few players involved in warm autoimmune hemolytic anemia clinical trials:
This section covers the detailed analysis of each drug under multiple phases including phase I, phase II, phase III, phase IV, and emerging drugs for warm autoimmune hemolytic anemia. It covers product description, trial ID, study type, drug class, mode of administration, and recruitment status of warm autoimmune hemolytic anemia (wAIHA) drug candidates.
Sponsored by Alexion Pharmaceuticals, Inc., the drug is under trials to assess its efficacy and safety in treating warm autoimmune hemolytic anemia (WAIHA). It is currently in Phase 2 of a randomised double-blind study.
As a part of the 24-week double-blind study, M281 is under examination for its efficacy and safety against warm autoimmune hemolytic anemia (wAIHA). It will be administered every 4 weeks during the 24-week double-blind period.
This oral warm autoimmune hemolytic anemia drug candidate is being evaluated to check the efficacy, safety and pharmacokinetics in patients. Sponsored by Sanofi, Rilzabrutinib is in Phase 2 of an open-label study.
Zenas BioPharma (USA), LLC is conducting a study to examine the safety and efficacy of obexelimab against warm autoimmune hemolytic anemia (wAIHA) in about 134 participants enrolled in the study.
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The Warm Autoimmune Hemolytic Anemia Drug Pipeline Insight Report provides a strategic overview of the latest and future landscape of treatments for warm autoimmune hemolytic anemia. It provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into warm autoimmune hemolytic anemia (wAIHA) collaborations, regulatory environments, and potential growth opportunities within the treatment landscape.




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
| Scope of the Report | Details |
| Drug Pipeline by Clinical Trial Phase |
|
| Route of Administration |
|
| Drug Classes |
|
| Leading Sponsors Covered |
|
| Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share