
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
The prevalence of uveitis and its associated complications is increasing globally, with the condition occurring in nearly 714 per 100,000 population and accounting for about 25% of all cases of blindness. Further, studies reveal that ongoing inflammation in untreated uveitis and complications linked to this uncontrolled inflammation are likely to be the reason behind 10% of the blindness in the United States. As a result, there is a growing interest in exploring effective treatment options that offer longer-lasting results and prevent vision loss in uveitis patients.
Major companies involved in the uveitis pipeline drugs market include Bio-Thera Solutions, Priovant Therapeutics, Inc., ACELYRIN Inc., and AbbVie.
Leading drugs currently under pipeline include Brepocitinib, QLETLI (Adalimumab Injection), and Izokibep, among others.
Pharmaceutical companies are increasingly investing in novel drug delivery systems and innovative treatments such as gene therapy and small-molecule inhibitors to target inflammation at the molecular level.
The Uveitis Drug Pipeline Report by Expert Market Research gives comprehensive insights into uveitis drugs currently undergoing clinical trials. It covers various aspects related to the details of each of these drugs under development for Uveitis. The report includes the analysis of over 100 pipeline drugs and 50+ companies. The uveitis pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials including their adverse effects on patients suffering from uveitis.
The assessment part will include a detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration, and ongoing uveitis pipeline development activities related to uveitis.
Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
Uveitis refers to an inflammatory disease that involves inflammation of the middle layer of the eye called uvea. The disease can be caused due to infections, idiopathic cases, trauma, and inflammatory diseases. The symptoms can range from redness, pain, and blurry vision to complete vision loss, indicating that prompt treatment is essential to prevent severe complications. Anterior uveitis ranks as the most prevalent type of the condition, comprising nearly 50% of all uveitis cases, whereas posterior uveitis is reported as the least common form.
Uveitis treatment depends on the underlying cause of the disease. Symptom-based treatments typically focus on pain control, inflammation reduction (steroids and nonsteroidal anti-inflammatory drugs (NSAIDs)), and condition-specific treatments. There is an increased emphasis on developing biologic drugs that target specific inflammatory pathways and new classes of small-molecule inhibitors, such as JAK inhibitors, in order to expand treatment options. Further, the rising investment in ophthalmic drug development and advancements in drug delivery technologies are driving the uveitis drug pipeline forward with several highly promising candidates in clinical development.
This section of the report covers the analysis of uveitis drug candidates based on several segmentations including:
By Phase
EMR’s pipeline assessment report covers 50+ drug analyses based on phase.
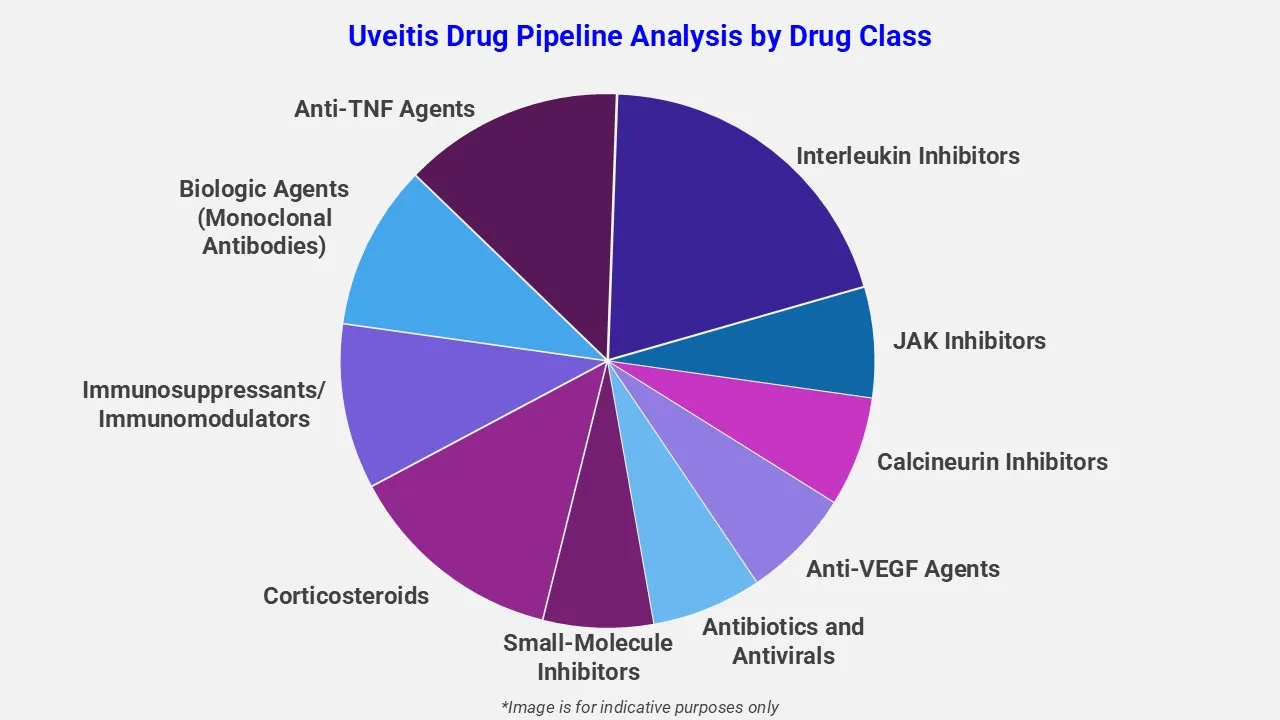
By Drug Class
EMR’s uveitis therapeutic assessment report covers 50+ drug analyses based on drug classes:
By Route of Administration
EMR’s pipeline assessment report covers 50+ drug analyses based on the route of administration.
The uveitis report covers phase I, phase II, phase III, phase IV, and early phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, phase II covers a major share of the total clinical trials for uveitis. There are around 109 drugs in phase II for uveitis.
The drug molecule categories covered under uveitis pipeline analysis are corticosteroids, immunosuppressants/immunomodulators, biologic agents, anti-TNF agents, interleukin inhibitors, jak inhibitors, calcineurin inhibitors, anti-VEGF agents, antibiotics and antivirals, and small-molecule inhibitors. The choice of treatment depends on multiple factors including the underlying inflammation and the immune response involved in the condition.
The EMR uveitis drug report insights cover the profile of key companies involved in clinical trials and their drugs under development. Below is the list of a few players involved in uveitis clinical trials:
This section covers the detailed analysis of each drug under multiple phases including phase I, phase II, phase III, phase IV, and emerging drugs for uveitis. It includes product description, trial ID, study type, drug class, mode of administration, and recruitment status of uveitis drug candidates.
Sponsored by Priovant Therapeutics, Inc., brepocitinib is a selective tyrosine kinase 2 (TYK2) and Janus kinase 1 (JAK1) inhibitor that is being investigated in a Phase 3 clinical trial with around 220 participants having active, non-anterior (intermediate, posterior, or pan) non-infectious uveitis (NIU).
QLETLI (Adalimumab Injection), Bio-Thera's biosimilar to Humira® is undergoing a Phase 4 post-marketing clinical study comprising around 60 participants suffering from uveitis. This prospective study aims to evaluate the clinical efficacy and safety of the drug for non-infectious uveitis.
Uveitis drug candidate Izokibep is in a Phase 2 study to evaluate its clinical activity against treating active non-infectious, intermediate-, posterior- or pan-uveitis that requires high-dose steroids. Developed by clinical biopharma ACELYRIN Inc., Izokibep is a small protein molecule that binds to interleukin-17A with high affinity, thereby acting as its selective, potent inhibitor.
Currently, in Phase 1 of a prospective, single-center phase 1 clinical study, Laquinimod eye drops are under investigation for their safety, tolerability, and distribution in 12 human participants for 2 weeks.
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The Uveitis Drug Pipeline Insight Report provides a strategic overview of the latest and future landscape of treatments for uveitis. It provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into uveitis collaborations, regulatory environments, and potential growth opportunities within uveitis pipeline insights.




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
|
Scope of the Report |
Details |
|
Drug Pipeline by Clinical Trial Phase |
|
|
Route of Administration |
|
|
Drug Classes |
|
|
Leading Sponsors Covered |
|
|
Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share