
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
Spinal fusion is a surgical procedure used to permanently connect two or more vertebrae in the spine, eliminating motion between them to relieve pain or correct deformities. According to Yubao Lu et al. (2024), the incidence of spinal cord injury (SCI) varies widely, ranging from 7 to 152.2 cases per million people. As per the Spinal Fusion Drug Pipeline Analysis by Expert Market Research, the market is witnessing significant advancements driven by the development of biologics, bone graft substitutes, and minimally invasive fusion therapies. Increasing prevalence of degenerative spinal disorders and rising R&D investments are expected to accelerate pipeline growth in the coming years.
The Spinal Fusion Pipeline Report by Expert Market Research gives comprehensive insights into ongoing spinal fusion clinical trials. It covers various aspects related to the details of spinal fusion drugs under development. The report includes the analysis of over 100 pipeline drugs and 50+ companies. The spinal fusion pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials including their adverse effects on patients suffering from spinal fusion.
The detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration and ongoing spinal fusion pipeline development activities are covered. Moreover, spinal fusion collaborations and commercial assessments are offered, helping stakeholders to make informed decisions.
Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
Spinal fusion involves the process of joining two or more spinal bones. The connection prevents movement and inhibits pain. During a spinal infusion surgery, a healthcare provider may use bonelike material, metal plates, screws or rods to hold bones together. This allows the bone to fuse and recover. Spinal fusion can help with the symptoms of degenerative disk disease, spinal stenosis, scoliosis, spondylolisthesis and tumor among others.
The spinal fusion treatment landscape involves the use of drugs and medications, that are often used to manage pain, and inflammation associated with the condition. In addition, bone growth stimulators and vitamin D supplements are also used to support bone health and expedite healing. Healthcare providers follow a multidisciplinary approach to optimize patient recovery and ensure procedure success. Currently, oxycodone and methocarbamol are commonly used in spinal fusion procedures. While oxycodone is an analgesic, methocarbamol is a skeletal muscle relaxant often prescribed post-operatively. Researchers are working on developing various drugs that help in manage the condition with higher efficacy.
This section of the report covers the analysis of spinal fusion drug candidates based on several segmentations including:
By Phase
EMR’s pipeline assessment report covers 50+ drug analyses based on phase:
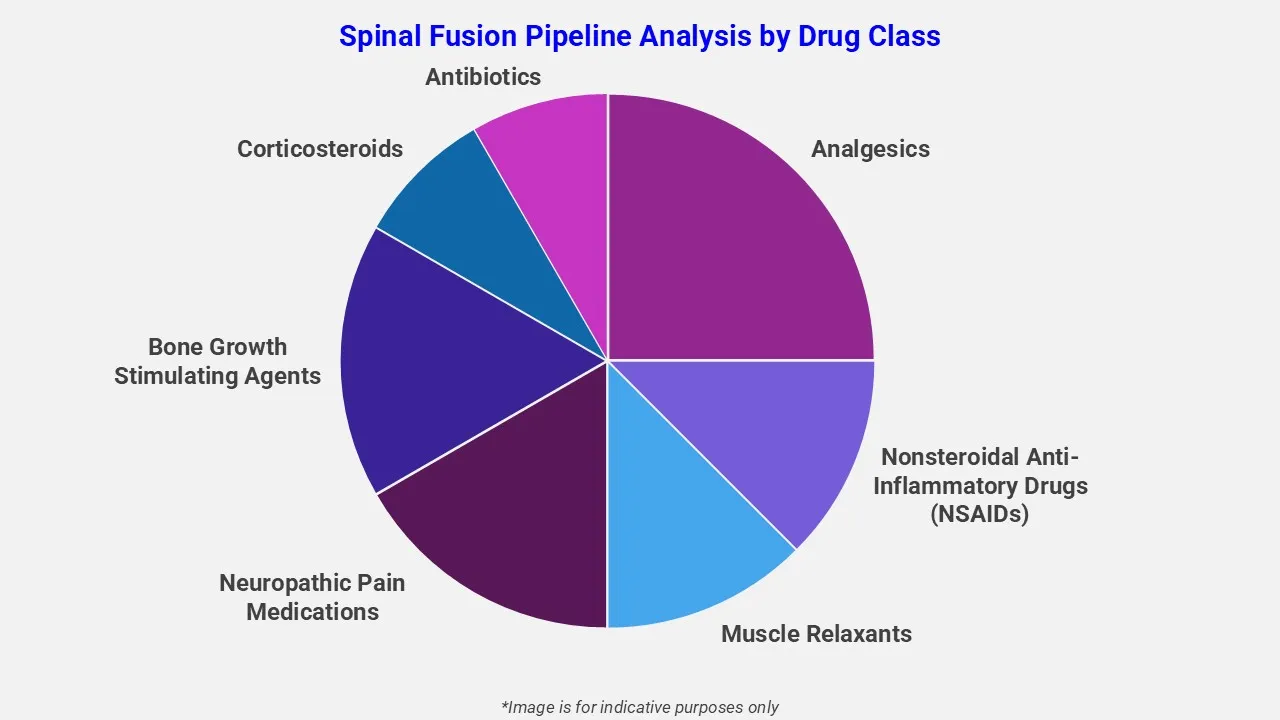
By Drug Class
EMR’s spinal fusion therapeutic assessment report covers 50+ drug analyses based on drug classes:
By Route of Administration
EMR’s pipeline assessment report covers 50+ drug analyses based on the route of administration:
The report covers phase I, phase II, phase III, phase IV, and early phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, phase IV covers a major share of the total spinal fusion clinical trials. There are around 65 drugs in phase IV of spinal fusion drugs.
The drug molecules categories covered under spinal fusion pipeline analysis include analgesics, nonsteroidal anti-inflammatory drugs (NSAIDs), muscle relaxants, neuropathic pain medications, bone growth stimulating agents, corticosteroids, and antibiotics. Each drug class has a specific function and is administered based on patient specifications.
The EMR spinal fusion drug report insights involve the profile of key companies involved in clinical trials and their drugs under development. Below is the list of a few players involved in spinal fusion clinical trials:
This section covers the detailed analysis of each drug under multiple phases including phase I, phase II, phase III, phase IV, and emerging drugs for spinal fusion. It includes product description, trial ID, study type, drug class, mode of administration, and recruitment status of spinal fusion drug candidates.
This is a neuropathic pain drug that is being tested to treat multi-modal pain regimen post-spinal surgery. Sponsored by the Hospital for Special Surgery, New York, the drug is currently in phase IV clinical trials.
Sponsored by the Ascension South East Michigan, this spinal fusion drug candidate is a low-dose ketorolac under investigation as an analgesic during the early post-operative period (within 48 hours) of spinal fusion.
Tranexamic acid is being evaluated as an alternative to reduce bleeding in individuals undergoing spinal fusion surgery. Sponsored by Exela Pharma Sciences, LLC, it is in Phase 3 of a multicenter, randomized, double-blind, parallel-group study.
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The Spinal Fusion Drug Pipeline Insight Report provides a strategic overview of the latest and future landscape of treatments for spinal fusion. It provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into spinal fusion collaborations, regulatory environments, and potential growth opportunities within the treatment landscape.




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
| Scope of the Report | Details |
| Drug Pipeline by Clinical Trial Phase |
|
| Route of Administration |
|
| Drug Classes |
|
| Leading Sponsors Covered |
|
| Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share