
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
Studies reveal that 9.5% of the global population is affected by liver fibrosis, with males having a higher prevalence of getting affected than females. The prevalence in males is around 10.6% while 5.4% of females may get develop the condition once in a lifetime. Moreover, the prevalence increases with age. Therefore, there is a significant emphasis on developing quality medications that help in disease management effectively.
Major companies involved in the liver fibrosis pipeline drugs market include Gilead Sciences, Intercept Pharmaceuticals and Bristol-Myers Squibb (BMS).
Leading drugs currently under pipeline include tirzepatide, and ALG-055009 among others.
Regulatory authorities such as the United States FDA and EMA play an essential role in liver fibrosis treatment as they are offering breakthrough designations to manage the condition.
The Liver Fibrosis Drug Pipeline Report by Expert Market Research gives comprehensive insights into liver fibrosis drugs currently undergoing liver fibrosis clinical trials. It covers various aspects related to the details of each of these liver fibrosis drugs under development for Liver fibrosis. The report includes the analysis of over 100 pipeline drugs and 50+ companies. The liver fibrosis pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials including their adverse effects on patients suffering from liver fibrosis.
The assessment part will include a detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration, and ongoing liver fibrosis pipeline development activities. Moreover, liver fibrosis collaborations and commercial assessments are offered, helping stakeholders to make informed decisions.
Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
Liver fibrosis involves the formation of scar tissues in large amounts, formed as a response to repairing and replacing damaged liver cells. There are different levels of liver fibrosis which are primarily based on the degree of liver damage. The condition does not have many symptoms in the mild to moderate stages. As the patient progresses, they may encounter symptoms like appetite loss, difficulty thinking clearly, fluid buildup in the legs or stomach, jaundice (where the skin and eyes appear yellow nausea, unexplained weight loss or weakness.
Nonalcoholic fatty liver disease (NAFLD) is the most common category of liver fibrosis, followed by alcoholic liver disease, which is due to long-term excesses of drinking alcohol. The liver fibrosis treatment varies based on the cause of illness and the symptoms of the patient. Some of the specific treatment alternatives include ACE inhibitors, direct-action antivirals, PPAR-alpha agonists and antifibrotics. Several universities and pharmaceutical companies are collaborating to develop high-efficacy drugs that manage the condition effectively. In March 2024, the United States FDA granted approval to Rezdiffra (resmetirom) for treating adults with noncirrhotic non-alcoholic steatohepatitis (NASH) with moderate to advanced liver scarring (fibrosis). Such approvals indicate a promising landscape for the drugs in the disease pipeline.
This section of the report covers the analysis of liver fibrosis drug candidates based on several segmentations including:
By Phase
EMR’s pipeline assessment report covers 50+ drug analyses based on phase.
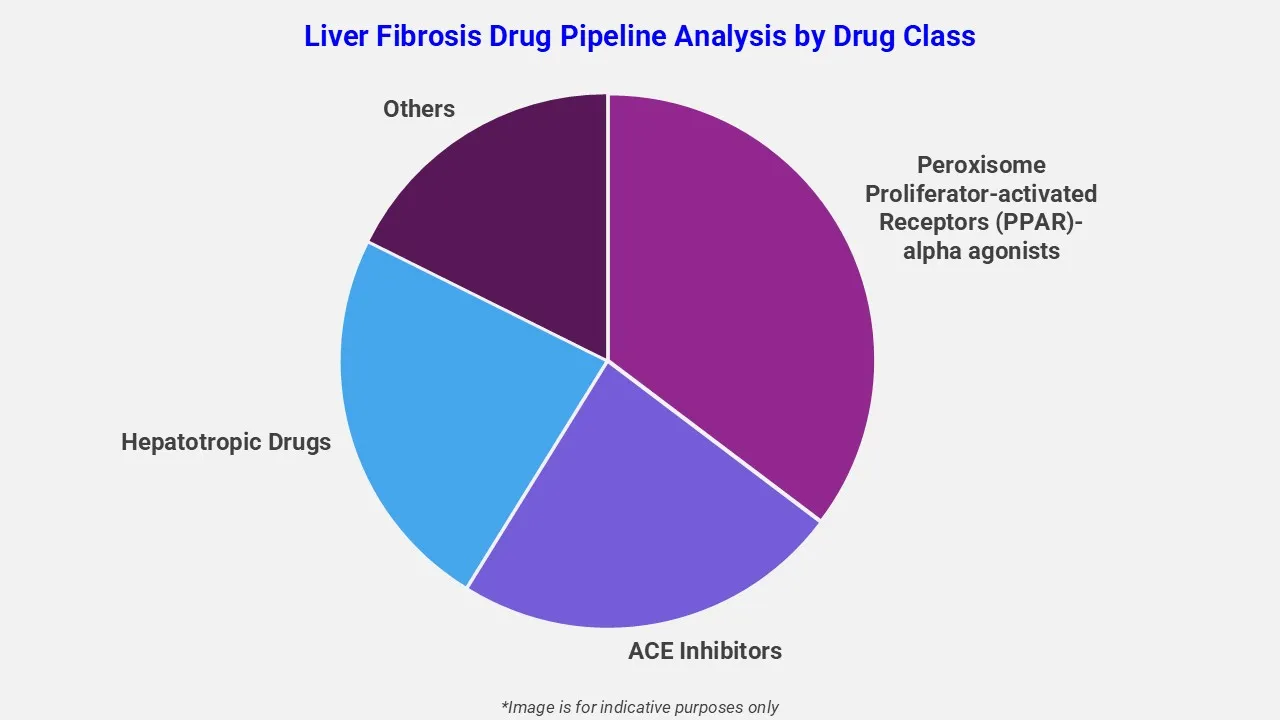
By Drug Class
EMR’s liver fibrosis therapeutic assessment report covers 50+ drug analyses based on drug classes:
By Route of Administration
EMR’s pipeline assessment report covers 50+ drug analyses based on the route of administration.
The liver fibrosis report covers phase I, phase II, phase III, phase IV, and early phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, phase II covers a major share of the total clinical trials for liver fibrosis. There are around 334 drugs in phase II of liver fibrosis drugs.
The drug molecule categories covered under liver fibrosis pipeline analysis include peroxisome proliferator-activated receptors (PPAR)-alpha agonists, ACE inhibitors, hepatotropic drugs and others. The report provides a comparative analysis of the drug classes for each drug in various phases of clinical trials for liver fibrosis.
The EMR liver fibrosis drug report insights cover the profile of key companies involved in clinical trials and their drugs under development. Below is the list of a few players involved in Liver fibrosis clinical trials:
This section covers the detailed analysis of each drug under multiple phases including phase I, phase II, phase III, phase IV, and emerging drugs for liver fibrosis. It includes product description, trial ID, study type, drug class, mode of administration, and recruitment status of liver fibrosis drug candidates.
Previously approved for treating type 2 diabetes, Eli Lilly's metabolic disorder medication tirzepatide, currently under phase 2 clinical trial of a 52-week study demonstrated high efficacy against nonalcoholic steatohepatitis (NASH) with no worsening of the liver fibrosis.
Aligos Therapeutics is conducting a Phase 2a study to investigate the safety, tolerability, efficacy, pharmacokinetics, and pharmacodynamics of oral (PO) daily (QD) doses of liver fibrosis drug candidate ALG-055009 (soft gelatin capsule) for 12 weeks. The study enrols 100 patients and is expected to be completed by December 2024.
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The Liver Fibrosis Drug Pipeline Insight Report provides a strategic overview of the latest and future landscape of treatments for liver fibrosis. It provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into liver fibrosis collaborations, regulatory environments, and potential growth opportunities within liver fibrosis pipeline insights.




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
|
Scope of the Report |
Details |
|
Drug Pipeline by Clinical Trial Phase |
|
|
Route of Administration |
|
|
Drug Classes |
|
|
Leading Sponsors Covered |
|
|
Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share