
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
Diarrhea is a condition characterized by the frequent passage of loose or watery stools, often caused by infections, food intolerance, or underlying health issues. Globally, diarrhea alone accounts for approximately 0.5 million deaths among children under the age of five annually and stands as the second most common cause of mortality in this age group. The diarrhea drug pipeline is witnessing significant advancements, with improved therapies and innovative diarrhea therapeutics under development. The growing focus on enhancing treatment efficacy, addressing antimicrobial resistance, and improving supportive care is expected to drive substantial growth in the pipeline.
Major companies involved in the diarrhea pipeline drug market include GlaxoSmithKline, Immuron Ltd., and others.
Leading drugs currently in the pipeline include Changkang Granules, Rifamycin SV-MMX, and others.
The rising global disease burden, increased research on novel therapeutics, and an emphasis on combating antimicrobial resistance are driving growth in the diarrhea drug pipeline.
The Diarrhe Drug Pipeline Insight Report by Expert Market Research gives comprehensive insights into diarrhea therapeutics currently undergoing clinical trials. It covers various aspects related to the details of each of these drugs under development for diarrhea. The diarrhea report assessment includes the analysis of over 100 pipeline drugs and 50+ companies. The diarrhea pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials, including their adverse effects on patients suffering from the condition, and alignment with diarrhea treatment guidelines to ensure optimal care practices.
The assessment part will include a detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration, and ongoing product development activities related to diarrhea.
Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
Diarrhea is a condition characterized by frequent, loose, or watery bowel movements. It occurs when the digestive system fails to absorb fluids properly or when excess fluids are secreted into the intestines. Causes include infections, food intolerance, medications, and digestive disorders. It can lead to dehydration if left untreated, especially in young children and older adults.
Diarrhea is treated by maintaining hydration through oral rehydration solutions (ORS) to prevent dehydration. Medications like loperamide may reduce symptoms, while antibiotics are prescribed for bacterial infections. Dietary adjustments, such as consuming bland foods, also aid recovery. In severe cases, intravenous fluids may be required.
Globally, diarrhea causes approximately 0.5 million deaths annually among children under five, making it the second leading cause of mortality in this group. Around 1.7 billion childhood diarrhea cases are recorded each year. In the United States, diarrheal illnesses account for 178.8 million cases annually, leading to 474,000 hospitalizations and 5,000 deaths. In Japan, chronic diarrhea affects approximately 3%-5% of the population, with higher prevalence among men, while India records 120,000 child deaths yearly.
This section of the report covers the analysis of diarrhea drug candidates based on several segmentations, including:
By Phase
By Drug Class
By Route of Administration
The report covers phase I, phase II, phase III, phase IV, and early-phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, phase II covers a major share of the total diarrhea clinical trials.
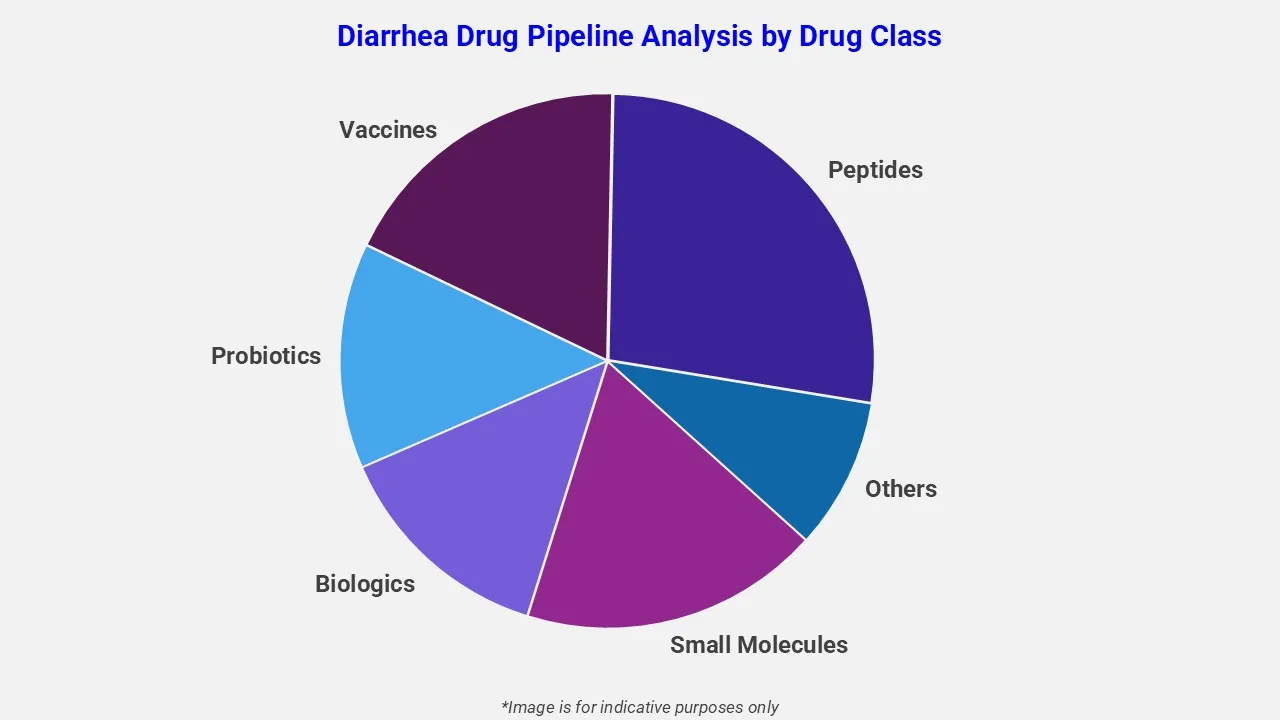
The drug molecule categories covered under the diarrhea pipeline analysis include small molecules, biologics, probiotics, vaccines, peptides, and others. The diarrhea report provides a comparative analysis of the drug classes for each drug in various phases of clinical trials for diarrhea.
The EMR report for the diarrhea drug pipeline covers the profile of key companies involved in clinical trials and their drugs under development. It provides a detailed diarrhea therapeutic assessment, analyzing the competitive dynamics of the clinical trial landscape. Below is the list of a few players involved in diarrhea clinical trials:
This section covers the detailed analysis of each drug under multiple phases, including phase I, phase II, phase III, phase IV, and emerging drugs for diarrhea. It includes product description, trial ID, study type, drug class, mode of administration, and recruitment status of diarrhea drug candidates.
A probiotic formulation is being investigated in a Phase IV clinical trial sponsored by Lallemand Health Solutions. The study aims to evaluate the safety and efficacy of a probiotic formulation in alleviating abdominal pain, abnormal defecation, anxiety, and depression and improving the quality of life in adults with irritable bowel syndrome with diarrhea (IBS-D). The trial is expected to be completed by November 2024
Changkang Granules, sponsored by Tasly Pharmaceutical Group Co., Ltd, is currently undergoing a Phase III clinical trial to evaluate its efficacy and safety in treating irritable bowel syndrome with predominant diarrhea. This randomized, double-blind, placebo-controlled study aims to assess the therapeutic potential of Changkang Granules, a traditional Chinese medicine, with an estimated completion date of December 2026.
Rifamycin SV MMX, marketed as Aemcolo®, is a non-systemic, delayed-release antibiotic designed to target the lower intestine using Cosmo Pharmaceuticals' MMX® technology for controlled drug delivery. Rifamycin SV MMX is currently being evaluated in a Phase II clinical trial, conducted by RedHill Biopharma Limited, to assess its safety and efficacy in treating traveler's diarrhea in children aged 6 to 11 years. This double-blind study involves 142 participants receiving either Rifamycin SV MMX or a placebo with oral rehydration therapy (ORT).
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The Diarrhea Drug Pipeline Insight Report provides a strategic overview of the latest and future landscape of treatments for diarrhea. It provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into diarrhea collaborations, regulatory environments, and potential growth opportunities.
Chemotherapy-induced Diarrhea Epidemiology Forecast
Traveler’s Diarrhea Market Report and Forecast




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
|
Scope of the Report |
Details |
|
Drug Pipeline by Clinical Trial Phase |
|
|
Route of Administration |
|
|
Drug Classes |
|
|
Leading Sponsors Covered |
|
|
Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share