
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
Cervical cancer starts at the cervix, the bottom portion of the uterus that joins the vagina. It usually arises from aberrant alterations in cervical cells, which are frequently brought on by a recurring infection with high-risk HPV strains. Squamous cell carcinoma, which originates from the outer cervix, and adenocarcinoma, which grows from glandular cells inside the cervix, are the two primary forms of cervical cancer. As the disease advances, symptoms may include odd discharge, pelvic pain, and abnormal bleeding, however the early stages may be asymptomatic. Frequent screening enables early intervention by identifying precancerous alterations. Cervical cancer pipeline analysis by Expert Market Research highlights promising drug candidates, including small molecules, gene therapies, and monoclonal antibodies.
Major companies involved in the cervical cancer pipeline analysis include Merck Sharp & Dohme LLC, Seagen Inc. and Iovance Biotherapeutics, Inc.
Leading drugs currently under the pipeline include pembrolizumab and tisotumab, among others.
The increasing cases of cervical cancer and the rising technological advancements are poised to positively influence the cervical cancer pipeline landscape.
The Cervical Cancer Pipeline Analysis Report by Expert Market Research gives comprehensive insights into cervical cancer therapeutics currently undergoing clinical trials. It covers various aspects related to the details of each of these drugs under development for cervical cancer. The cervical cancer report assessment includes the analysis of over 25 pipeline drugs and 10+ companies. The cervical cancer pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials including their adverse effects on patients suffering from the condition, and alignment with cervical cancer treatment guidelines to ensure optimal care practices.
The assessment part will include a detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration, and ongoing product development activities related to cervical cancer.
Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
High-risk human papillomavirus (HPV) infections, especially those of strains 16 and 18, are the main cause of cervical cancer. The infection causes alterations in the cervix's cells, which eventually lead to invasive cancer from cervical intraepithelial neoplasia (CIN). By deactivating tumor suppressor proteins including p53 and pRB, HPV oncoproteins E6 and E7 promote unchecked cell proliferation and interfere with normal cell cycle regulation. Carcinogenesis can be further aided by environmental factors and host immunological state, which can result in the development and progression of tumors.
Depending on the stage and features of the cancer, treatment for cervical cancer usually consists of a mix of radiation therapy, chemotherapy, and surgery. Hysterectomy and radical trachelectomy are surgical procedures used to remove malignant tissue. To increase effectiveness, especially for advanced stages, chemotherapy can be given either by itself or in combination with radiation. After surgery, radiation therapy is frequently used to lower the risk of recurrence. It can be internal (brachytherapy) or external. For more severe instances, new medicines like immunotherapy and targeted therapies are also being investigated. To manage side effects and track healing, routine follow-ups are crucial.
Globally, cervical cancer ranks fourth in terms of incidence among women. In 2022, there were around 660,000 new cases and 350,000 fatalities. Women are disproportionately affected by the disease in low- and middle-income nations, where 94% of fatalities are attributable to a lack of access to HPV screening, treatment, and immunization. The largest incidence rates are found in Central America, Southeast Asia, and Sub-Saharan Africa. Cervical cancer is six times more common in women living with HIV. Disparities by region emphasize the necessity of fair access to healthcare.
This section of the report covers the analysis of cervical cancer drug candidates based on several segmentations including:
By Phase
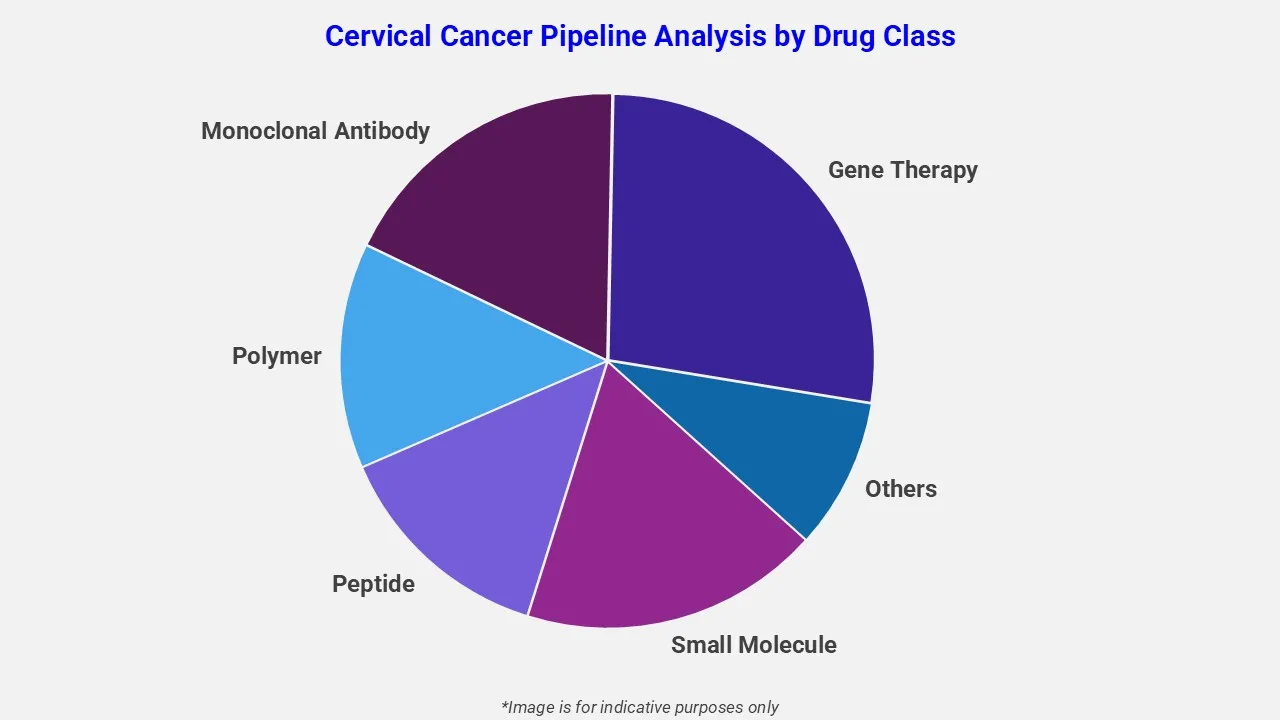
By Drug Class
By Route of Administration
The report covers phase I, phase II, phase III, phase IV, and early phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, phase III covers a major share of the total clinical trials, with a substantial number of cervical cancer drugs undergoing clinical development.
The drug molecule categories covered under cervical cancer pipeline analysis include small molecules, biologics, peptides, and immunotherapies, among others. The cervical cancer report provides a comparative analysis of the drug classes for each drug in various phases of clinical trials for cervical cancer.
The EMR report for the cervical cancer pipeline covers the profile of key companies involved in clinical trials and their drugs under development. It provides a detailed cervical cancer therapeutic assessment, analyzing the competitive dynamics of the clinical trial landscape. Below is the list of a few players involved in cervical cancer clinical trials:
This section covers the detailed analysis of each drug under multiple phases, including phase I, phase II, phase III, phase IV, and emerging drugs for cervical cancer. It includes product description, trial ID, study type, drug class, mode of administration, and recruitment status of cervical cancer drug candidates.
Merck Sharp & Dohme LLC created the humanized monoclonal antibody pembrolizumab, which is sold under the brand name Keytruda. It increases the immune system's capacity to combat cancer cells by targeting the PD-1 receptor. Cervical cancer is among the many cancers for which the FDA has authorized it. For locally advanced cervical cancer, pembrolizumab is presently undergoing Phase 3 clinical research to evaluate its safety and effectiveness when used in conjunction with concurrent chemoradiotherapy. The primary goals of the experiment are to assess overall survival and progression-free survival.
Tisotumab vedotin is an antibody-drug conjugate (ADC) created by Seagen to target tissue factor (TF), a protein that is expressed in a variety of solid tumors, including cervical cancer. It causes cancer cells to die by delivering a cytotoxic substance. Patients with recurrent or metastatic cervical cancer who have not responded to prior treatments are presently enrolled in a Phase 2/3 clinical trial to assess the medication's safety and effectiveness. The primary endpoints of the experiment are progression-free survival and overall survival.
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The Cervical Cancer Pipeline Analysis provides a strategic overview of the latest and future landscape of treatments for cervical cancer. it provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into market trends, regulatory environments, and potential growth opportunities within the cervical cancer pipeline insights.




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
|
Scope of the Report |
Details |
|
Drug Pipeline by Clinical Trial Phase |
|
|
Route of Administration |
|
|
Drug Classes |
|
|
Leading Sponsors Covered |
|
|
Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share