
Consumer Insights
Uncover trends and behaviors shaping consumer choices today
Procurement Insights
Optimize your sourcing strategy with key market data
Industry Stats
Stay ahead with the latest trends and market analysis.
As per the estimates from CDC's (Centers for Disease Control and Prevention) Autism and Developmental Disabilities Monitoring (ADDM) Network, around 1 in 36 children were reported to be identified with autism spectrum disorder. With the growing number of cases, there is a heightened focus on expediting the development of promising autism drugs and accelerating clinical trials. The autism pipeline analysis by Expert Market Research includes major drugs currently under development. Moreover, rising public awareness and advocacy from autism organizations are also fueling the demand for advanced treatment options.
Major companies involved in the autism pipeline drugs market include Curemark, Astrogen, Inc., and ACADIA Pharmaceuticals Inc., among others.
Leading drugs currently under the pipeline include CM-AT and AST-001, among others.
The increasing prevalence of autism along with the rising advancements in neurological and genetic research are poised to positively influence the autism pipeline landscape.
The Autism Pipeline Analysis Report by Expert Market Research gives comprehensive insights into autism therapeutics currently undergoing clinical trials. It covers various aspects related to the details of each of these drugs under development for Autism. The autism report assessment includes the analysis of over 100 pipeline drugs and 50+ companies. The autism pipeline landscape will include an analysis based on efficacy and safety measure outcomes published for the trials including their adverse effects on patients suffering from the condition, and alignment with autism treatment guidelines to ensure optimal care practices.
The assessment part will include a detailed analysis of each drug, drug class, clinical studies, phase type, drug type, route of administration, and ongoing product development activities related to autism.
Read more about this report - REQUEST FREE SAMPLE COPY IN PDF
Autism or autism spectrum disorder (ASD) refers to a neurological and developmental disorders characterized by deficits in social communication and social interaction. Autistic individuals typically show inflexible, repetitive, and restricted patterns of interests, behaviors, and activities. Some people with autism can live independently whereas certain individuals may require life-long support and care.
Behavioral therapy is a widely used approach to improve social, communication, and learning skills in autistic people. For irritability and aggression, autism therapeutics include FDA-approved risperidone and aripiprazole for managing severe behavioral symptoms. Pharmaceutical companies are actively developing novel drug candidates such as gene and RNA-based therapies, cannabidiol (CBD), and oxytocin therapies. Additionally, the adoption of innovative technologies in drug development, along with adherence to autism treatment guidelines, is expected to improve patient outcomes and aid pipeline expansion in the near future.
According to the World Health Organisation (WHO ), approximately 1 in 100 children are affected by autism, with the prevalence of the condition unknown in many low- and middle-income countries. On the other hand, the CDC's Autism and Developmental Disabilities Monitoring (ADDM) Network estimates that 1 in 36 children has been identified with autism.
Autism spectrum disorder is roughly 4 times more common in boys than in girls. It is reported to occur in all ethnic, racial, and socioeconomic groups.
This section of the report covers the analysis of autism drug candidates based on several segmentations including:
By Phase
The pipeline assessment report covers 50+ drug analyses based on phase:
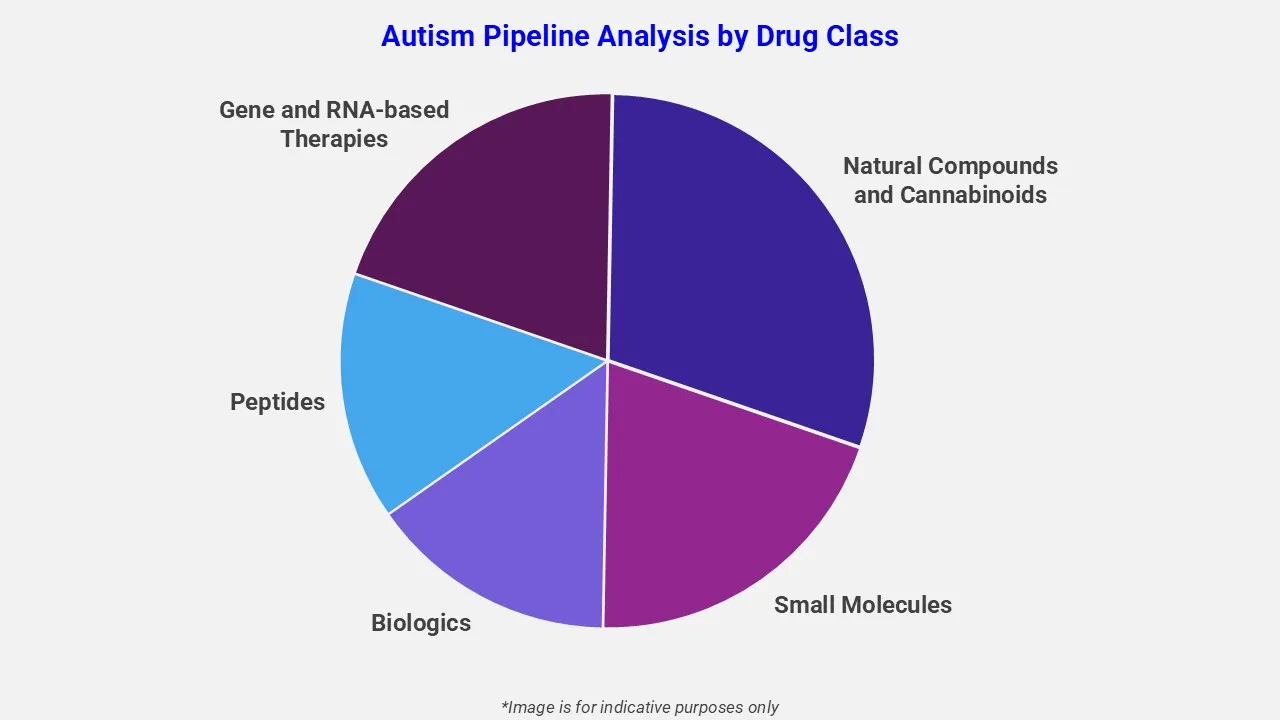
By Drug Class
The pipeline assessment report covers 50+ drug analyses based on drug classes:
By Route of Administration
The autism report assessment covers 50+ drug analyses based on the route of administration.
The report covers phase I, phase II, phase III, phase IV, and early phase drugs. The coverage includes an in-depth analysis of each drug across these phases. According to EMR analysis, phase II covers a major share of the total clinical trials, with a substantial number of autism emerging drugs undergoing clinical development. Phase II holds the largest share at 50%, demonstrating strong progress in syphilis drug development. Phase I follows at 15%, reflecting early-stage advancements. Phase III also accounts for 15%, indicating promising late-stage trials. Phase IV contributes 5%, supporting post-market evaluation. Early Phase I at 3% highlights emerging innovations. These developments can drive market growth and improve treatment outcomes.
The drug molecule categories covered under the autism pipeline analysis include small molecules, biologics, peptides, gene and RNA-based therapies, and natural compounds and cannabinoids. The autism report provides a comparative analysis of the drug classes for each drug in various phases of clinical trials for autism. Endocannabinoid system modulators are emerging as a promising drug class in autism spectrum disorder (ASD) treatment. For instance, KT-20610, an orally administered therapy by Kingdom Therapeutics, is under investigation. It targets the gut-brain axis by modulating the endocannabinoid system, aiming to restore physiological balance and address core ASD symptoms while utilizing a delivery method that enhances bioavailability and reduces adverse effects.
The EMR report for the autism drug pipeline covers the profile of key companies involved in clinical trials and their drugs under development. It provides a detailed autism therapeutic assessment, analyzing the competitive dynamics of the clinical trial landscape. Below is the list of a few players involved in autism clinical trials:
This section covers the detailed analysis of each drug under multiple phases, including phase I, phase II, phase III, phase IV, and emerging drugs for vitiligo. It includes product description, trial ID, study type, drug class, mode of administration, and recruitment status of autism drug candidates.
Sponsored by Astrogen, Inc., the objective of this randomized, double-blinded, multicenter clinical trial is to investigate the efficacy and safety of AST-001 in children with autism spectrum disorder. The study is under Phase III clinical development and has an estimated 160 participants.
Curemark is conducting a Phase III open-label extension study aimed at examining the efficacy and safety of autism drug candidate CM-AT in treating autism with all levels of fecal chymotrypsin. The interventional study has enrolled about 405 children and is expected to be completed by January 2025.
*Please note that this is only a partial list; the complete list of drugs will be available in the full report.*
The Autism Pipeline Analysis Report provides a strategic overview of the latest and future landscape of treatments for autism. It provides necessary information for making informed investment decisions along with research, development, and strategic planning efforts. The stakeholders will benefit from the essential insights into market trends, regulatory environments, and potential growth opportunities within autism pipeline insights.




*While we strive to always give you current and accurate information, the numbers depicted on the website are indicative and may differ from the actual numbers in the main report. At Expert Market Research, we aim to bring you the latest insights and trends in the market. Using our analyses and forecasts, stakeholders can understand the market dynamics, navigate challenges, and capitalize on opportunities to make data-driven strategic decisions.*
Get in touch with us for a customized solution tailored to your unique requirements and save upto 35%!
Explore our key highlights of the report and gain a concise overview of key findings, trends, and actionable insights that will empower your strategic decisions.
|
Scope of the Report |
Details |
|
Drug Pipeline by Clinical Trial Phase |
|
|
Route of Administration |
|
|
Drug Classes |
|
|
Leading Sponsors Covered |
|
|
Geographies Covered |
|
Mini Report
One User
USD 1,999
USD 1,799
tax inclusive*
Single User License
One User
USD 3,099
USD 2,789
tax inclusive*
Five User License
Five User
USD 4,599
USD 3,909
tax inclusive*
Corporate License
Unlimited Users
USD 5,999
USD 5,099
tax inclusive*
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Small Business Bundle
Growth Bundle
Enterprise Bundle
*Please note that the prices mentioned below are starting prices for each bundle type. Kindly contact our team for further details.*
Flash Bundle
Number of Reports: 3
20%
tax inclusive*
Small Business Bundle
Number of Reports: 5
25%
tax inclusive*
Growth Bundle
Number of Reports: 8
30%
tax inclusive*
Enterprise Bundle
Number of Reports: 10
35%
tax inclusive*
How To Order

Select License Type
Choose the right license for your needs and access rights.

Click on ‘Buy Now’
Add the report to your cart with one click and proceed to register.

Select Mode of Payment
Choose a payment option for a secure checkout. You will be redirected accordingly.
Gain insights to stay ahead and seize opportunities.

Get insights & trends for a competitive edge.

Track prices with detailed trend reports.

Analyse trade data for supply chain insights.

Leverage cost reports for smart savings

Enhance supply chain with partnerships.

Connect For More Information
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
Our expert team of analysts will offer full support and resolve any queries regarding the report, before and after the purchase.
We employ meticulous research methods, blending advanced analytics and expert insights to deliver accurate, actionable industry intelligence, staying ahead of competitors.
Our skilled analysts offer unparalleled competitive advantage with detailed insights on current and emerging markets, ensuring your strategic edge.
We offer an in-depth yet simplified presentation of industry insights and analysis to meet your specific requirements effectively.
Share